And if you could keep the impurities from contaminating it you could put a radio in it and it would still work. H2g COg CH2Og H 19 kJ.
Distilled Tap Sea And Rain Water Chemical Effect Of Electric Current Class 8
Conductors in their solution form are called ionic conductors.

. Describe how water can be a good conductor of current C A use pure water B heat from CHM 201 at William Rainey Harper College. Water acts as a polar solvent because it can be attracted to either the positive or negative electrical charge on a solute. Vegetable oil No Poor Conductor 5.
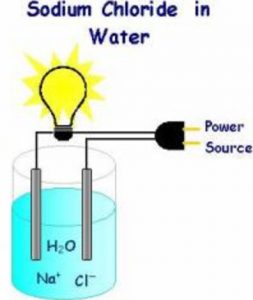
Liquid in which ionic compounds can dissolve easily. Salts and impurities conduct electricity. The ordinary water can conduct electricity while distilled water does not.
C N2g3 Determine Grxn using the following information. So by the addition. E Add a soluble salt.
Pure water is actually a good insulator. Why not make sure youre using the right one. However water is a great ionic solvent.
A use pure water B Heat the Water C add salt D Chill the water E Vaporize the water. The water that we get from sources such as taps hand pumps wells and ponds is not pure. S -1096 JKE 346 kJWhat is the.
Tap Water Yes Good Conductor 4. If we attach wires to the metal part of the screwdriver the bulb will glow. Distilled water can be made a conductor of electricity by adding salts and impurities.
E add saltIdentify the species with the standard free energy of formation equal to zero. D Chill the water. The coil or wire shows magnetism till current is passed.
Describe how water can be a good conductor of current. The only reason water can sometimes conduct electricity is because of the minerals metallic solids already present in the water. When you put salt in water the salt dissolved into solution.
Water molecules pull the sodium and chlorine ions apart so. Distilled water can be made good conductor by adding some salt acids and gases. How can distilled water be made conductor of electricity.
C Vaporize the water. Liquids like Distilled Water Alcohol do not conduct electricity. Chemistry questions and answers.
B Heat up the water. Pure water is a bad conductor of electricity. The slight negative charge near the oxygen atom attracts nearby.
For example saltwater is an ionic solution. Water is indeed NOT a good conductor UNLESS impurities exist. Good ConductorPoor Conductor 1Lemon juice Yes Good Conductor 2Vinegar Yes Good Conductor 3.
The water that we get from. 73 pts Describe how water can be a good conductor of electricity a Use pure water. Shaun Gladman Student BHS.
So it is a good conductor of electricity. Lets face it water words are something that will come up in your writing at least once. Because it contains some dissolved calcium and magnesium salts which are good conductors of electricity.
Salt molecules are made of charged sodium ions and chlorine ions. Water is a terrible conductor of electricity. However most non-metals are not good conductors of electricity.
Describe how water can be a good conductor of current.

Does Water Conduct Electricity Simple Experiment

Everyday Life If Water Is Not A Good Conductor Why Are We Advised To Avoid Water Near Electricity No Wet Hands Near Circuits Etc Physics Stack Exchange
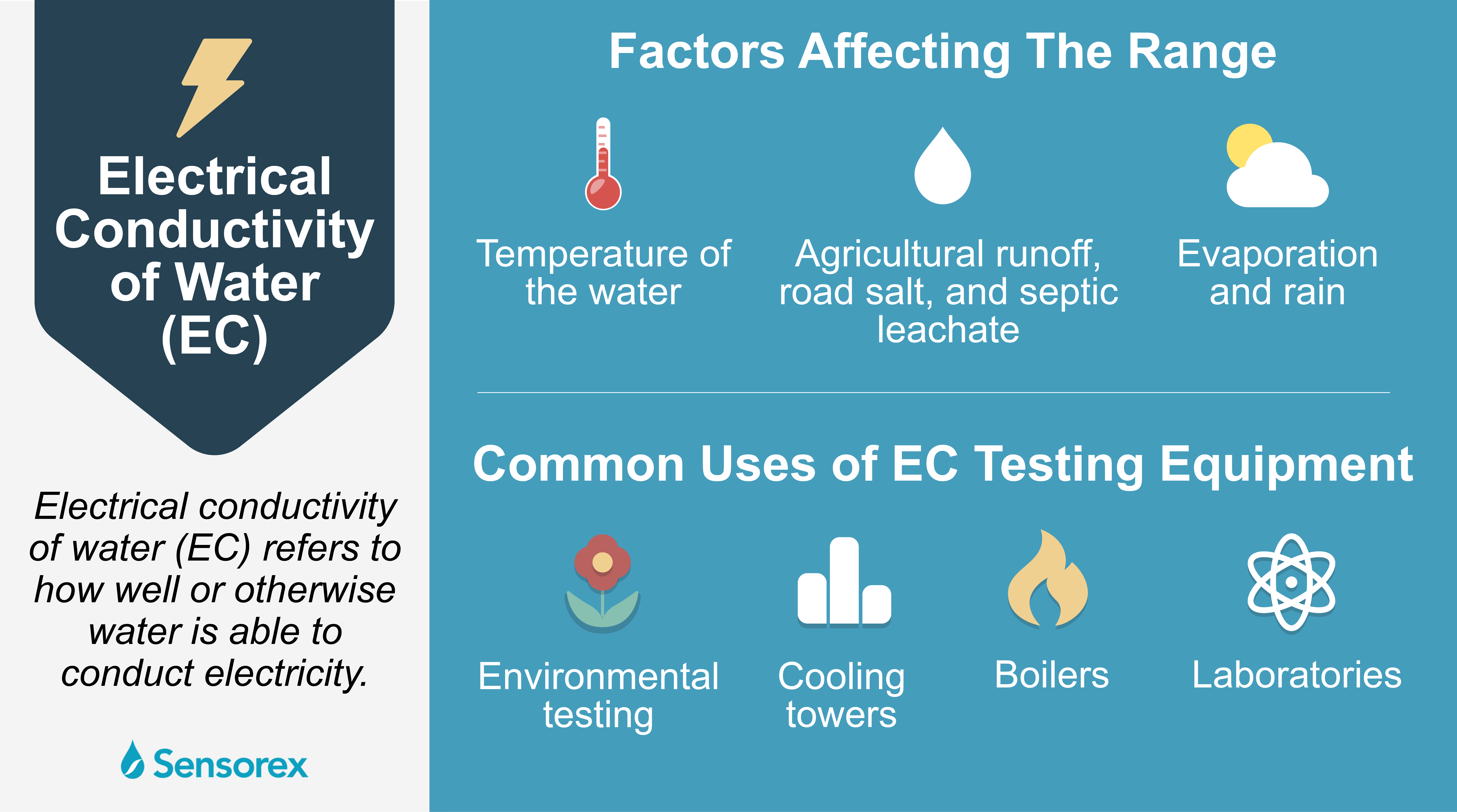
Why Electrical Conductivity Of Water Is Important For Industrial Applications Sensorex

Pin By Elveena Elle On Lessons Current Electric Electrical Conductor Electric Circuit
0 Comments